Vascular Access and Haemodialysis Study Day
27th September 2024
The paper under review investigates the utility of the Wells score for risk stratification of suspected Deep Vein Thrombosis (DVT) in hospitalised patients. The authors compared their findings with those of the Wells et al1 (1997) paper, which validated the Wells score in outpatient and emergency department settings. The purpose of the paper was to determine if the Wells score is of utility in stratifying risk of DVT in populations who develop DVT symptoms whilst under in-patient admission.
This was a prospective study, undertaken at a quaternary hospital in the United States. All inpatients with suspected DVT who had a lower limb venous duplex ultrasound between the 1st November 2012 and 31st December 2013 were enrolled. Patients with a history of DVT in the prior three months were excluded. If the patient underwent multiple lower limb venous duplex examinations in the period, only the first was included. In total, 1135 patients were enrolled.
For the purpose of this study, the requesting physician or physician’s assistant completed an automated check list of the Wells criteria when requesting an ultrasound examination. Both the ordering and interpreting clinicians were blinded to the final Wells score.
The Wells score is calculated as follows. The patient receives 1 point for each of the following:
Finally, the patient receives a -2 to the total score if
The totalled score is then used to stratify the patient into three categories: low, moderate, and high pre-test probability of having a proximal DVT, corresponding to Wells scores of ≤0, 1 to 2, and >2 respectively.
The results (for proximal DVT) broken down by Wells stratification, are given in table 1:
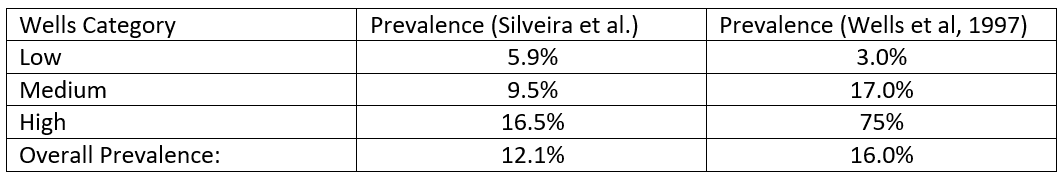 |
Table 1. Prevalence of DVT by Wells stratification in Silveira et al (2015) and Wells et al (1997)
The predictive Wells score was compared to the ultrasound findings and statistical analysis was performed using an area under the (receiver operating characteristics) curve methodology in which true positive rates are plotted against the false positive rates. If the Wells score is optimally predictive the area under the curve would equal 1.0 and if the scoring system has no ability to discriminate, the area under the curve would be 0.5. The study found the area under the curve was 0.6, only slightly better than chance.
Additionally, the authors of this paper calculated the failure rate and efficiency of the Wells score. The failure rate was defined as the mean predicted probability of DVT with a low probability Wells score. The efficiency is the proportion of patients in the low probability group.
In the in-patient study, the failure rate was 5.9%, almost double that quoted by Wells et al. (3%). The efficiency of the Wells score in the in-patient setting was only 11.9%, compared with 55.5% in Wells et al.
From this the authors posited a number of reasons for the apparent discrepancy in performance for the Wells score in stratifying proximal DVT risk when comparing inpatients with outpatients. They identified inpatients as having a greatly increased prevalence of cancer, prolonged bed rest, and recent surgery in comparison to the general population. They also have an increased prevalence of known risk factors for DVT (eg. chronic obstructive pulmonary disease, heart failure, acute infection, and peripheral arterial disease), none of which are explicit in the Wells scoring system. Most interestingly, 57% of patients in this study were receiving thromboprophylaxis, an exclusion criterion for the original Wells research.
The paper raises a number of questions. All ultrasound examinations in the study were graded as either positive or negative for DVT with no equivocal results. According to recent US government data2, over 40% of the adult US population are classified as obese (BMI>30) and approximately 10% severely obese (BMI>40). From other research3 there is evidence of a linear correlation between BMI and suboptimal ultrasound imaging quality and this certainly conforms to my own clinical experience as a vascular sonographer. Therefore I find it surprising that there is no report of sub-optimal or insufficiently diagnostic results within the study.
Also of interest, the inpatient population in this study shows a lower prevalence of DVT than Wells’ outpatient study (as demonstrated in table 1). This is despite a higher number of thrombosis predisposing factors in the inpatient population. Is thromboprophylaxis alone sufficient to explain this discrepancy?
The authors of this study concluded that ‘the Wells score only performed slightly better than chance for discriminating the risk of DVT in hospitalised patients’ and that ‘the Wells score risk stratification is not sufficient to rule out DVT or influence management decisions in the in-patient setting’.
On the basis of the research presented here, it is clear that the Wells score is of limited utility in the in-patient setting, with only one in eight categorised as low risk. This is unsurprising given the number of DVT risk factors present in the in-patient population.
In conclusion, calculation of the Wells score is unlikely to reduce the demand on in-patient ultrasound services or significantly alter patient management.
Simon Hartley, AVS
Sheffield Teaching Hospitals Foundation Trust